Lake Erie's future - look, think, and hope
Lake Erie's future - look, think, and hope
By Dave Adams
While fishing, by email, at seminars, and from editors, I am often asked about the ecology of Lake Erie. Many of those questions include complicated biological situations such as fish to prey balance, zebra mussels, gobies, and pollution. Rarely, do I have an instant answer. Instead, what I usually do is - look, think, and hope. I look at the past, I think about what is happening now, and I hope that we don't make the same mistake twice. The article below is long - and at times, somewhat boring - but it looks at a past mistake and what is happening now, with the hope that we don`t make the same mistake, twice. Thank You,
Capt. Dave Adams Have we become so comfortable with our quality of life, that we have forgotten that parallels are drawn to chemicals, organic and inorganic that have benefited us; yet, years later are proven harmful?
"If Darwin were alive today the insect world would delight and astound him with its impressive verification of his theories of the survival of the fittest," Rachel Carson said in her 1962 book, Silent Spring, which launched the modern environmental movement and the ban of DDT.
 That movement is continuing and needed. Because, currently, too much phosphorus is being dumped into Lake Erie, and its result is as damaging as DDT was to our country's waters and food chain.
That movement is continuing and needed. Because, currently, too much phosphorus is being dumped into Lake Erie, and its result is as damaging as DDT was to our country's waters and food chain. Paul Muller of Geigy Pharmaceutical in Switzerland was awarded the 1948 Nobel Prize in medicine and physiology after he discovered DDT's (dichloro-diphenyl-trichloro-ethane) effectiveness as an insecticide. The World Health Organization estimated its use, shortly after World War II, saved 25 million lives because of its ability to kill mosquitoes, which spread malaria. DDT was a cure-all for every insect problem. If an insect pest began interfering with crop production, a single application of DDT would destroy the insect population and solve the problem. Its use saved lives and increased crop yields. The end result, however, was worse. The insect pests were eliminated, but also it destroyed the birds, which prey on those insects. As a result, DDT would increase yields; but later, decrease yields because pest insects were far more capable of recovering from the effects of DDT than were their natural predators. The United States banned DDT in 1973. That experience taught us that science can help the natural order of life, yet it was a lesson in the sensitivity of our most precious and valuable resource of all, water, and its role in the food chain. Phosphorus is having the same effect. "High availability of phosphorus decreases Lake Erie water quality," Ohio State University ecology professor, David Culver, said, July 28, 2002, in his testimony during a U.S. Senate hearing held in Cleveland, Ohio, on the appearance of a large area in middle Lake Erie that had become anoxic (lacking enough oxygen to support all fish life), or commonly referred to as the late-summer natural phenomenon known as the "dead zone" in Lake Erie.
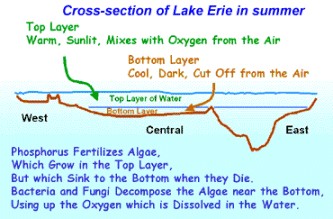
Courtesy of EPA
Phosphorus is a nutrient needed by all organisms for the basic processes of life, and is a natural element found in rocks, soils and organic material. In natural waters it is usually found in the form of phosphates. Phosphates can be in inorganic form (including orthophosphates and polyphosphates), or organic form (organically bound phosphates). Inorganic phosphate is phosphate that is not associated with organic material. Types of inorganic phosphate include orthophosphate and polyphosphates. Orthophosphate is the most stable phosphate, and is the form used by plants. Orthophosphate is produced by natural processes and is found in sewage and used in fertilizers. Polyphosphates are used in detergents. All of which, when used too much, alters the food chain in natural waters. Natural waters have a phosphorus concentration of approximately 0.02 parts per million (ppm). Above that, toxic blue-green algae and "algae blooms" will form. "Toxic algae tend to float to the surface in later summer and can be blown to shore," said Culver, "increasing the likelihood they will be taken in by potable water intakes and causing risks for swimmers, and for wildlife, livestock, and pet animals that may drink from the shore of the lake. Toxic algae have been shown to negatively affect the food chain upon which fish depend." Previously, high phosphorus levels in Lake Erie, caused by fertilizers, laundry detergent and human sewage caused a large outbreak of blue-green algae in the 1970s. After controls to limit phosphates, it disappeared in the late 1980s, until 1995.
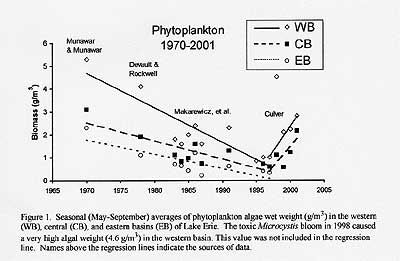
Courtesy of David Culver
And phosphorus levels in Lake Erie have been rising since, and algae have been increasing since 1997. Scientists say if this continues for another three years, Lake Erie could face the same problems it did in the 1970s. But this time, the solution is complicated. Because phosphorus is not a regulated pollutant, and other sources contribute to the loading (weight of phosphorus added to a water). For example, billions of gallons of untreated water flow into Lake Erie each year. "The amount of phosphorus this water contributes to Lake Erie is astonishing," said Elaine Marsh, board member of Great Lakes United, to Senator George Voinovich of Ohio after her testimony in the 2002 anoxia hearing. "And (during heavy rains) along certain stretches of the Ohio and Erie Canal Towpath Trail, one of the nation's most visited trails, the smell of sewage is overwhelming." During heavy rains (such as this year) overflow releases of partially treated sewage occurs through a combined sewer system overflow (CSO) or a sanitary sewer overflow (SSO). Separate sanitary sewers are designed to carry only sanitary sewage to a waste water treatment plant, storm water is directed to a nearby river, lake or stream via storm sewers. Combined sewer systems are older sewer systems designed to carry sewage and storm water to a waste water treatment plant. CSOs are overflows from older combined sewer systems designed to carry both sanitary sewage and storm water. SSOs are discharges of raw or inadequately treated sewage from municipal sanitary sewer systems, which are designed to carry sanitary sewage but not storm water. These overflows discharge untreated human and industrial waste. Another cause of increased phosphorus is the zebra mussel. "Zebra mussels have recycled phosphorus and nitrogen in algae that otherwise would have settled to the sediments and stayed there," Culver said. "They consume algae all year round, providing continuous recycling of nutrients that can encourage algae growth. Their effects will be particularly felt in the western basin and near shore, but these waters also flow into the central basin." So, is it time to ban phosphorus? As with any major change in way of life, though, that might not be possible. Phosphorus is an important part of our life. Plants require large quantities of phosphorus. In an average year, the crops of corn, wheat and alfalfa, in the U.S, remove nearly 1.5 billion pounds of phosphorus from the soil. And it must be restored or crops will not produce. "But if the goal is a healthy Lake Erie," said Marsh, "we should control phosphorus." But how much can a body of water handle? The goal for Lake Erie, set in 1985, was 11,000 metric tons (approximately 2,200 pounds in the English system of measurements) per year. That was thought, by the Environmental Protection Agency (EPA), to be the maximum carrying capacity for a healthy lake, and to avoid a repeat of the previous problems. Now the EPA is thinking that a 6,000 ton limit is needed. What can be done to achieve lower limits? The United States, currently, has a 0.5% limit of phosphorus by weight in laundry detergent. Phosphate, however, is still permitted in dishwashing detergents and commercial cleaning agents as well as fertilizers. In fact, one common brand-name dish detergent, contains 4.5% phosphorus in the form of phosphates, which is equivalent to 1.0 grams per tablespoon, and a common plant food, used for home gardening, has a phosphorus level of 30%. When household use is multiplied by everyone in the Lake Erie watershed, which is about one-third of the total Great Lakes basin, that 0.5% limit is absurd because an incredible amount of phosphorus is released into the watershed. "In the 60`s and 70s` excess phosphorus was a major source of Lake Erie`s decline," said Dr. Paul Bertram, Environmental Scientist with the EPA. But with the enactment of the Clean Water Act there was a marked improvement." "But now, we are not winning the battle on phosphorus," Bertram said. "In the springtime of 1990 we started to notice an increase in phosphorus loading." State fish agencies agree that monitoring of phosphorus loading is the best way to prevent another environmental catastrophe on Lake Erie. "You can not totally eliminate phosphorus, but you can monitor it to be sure that it is not too concentrated," Kevin Kayle, aquatic biologist for the Ohio DNR, Division of Wildlife said. "More of a concern is how exotic species change the way everything is being recycled." "You can monitor phosphates in the water," Pennsylvania Fish and Boat Commission fisheries biologist, Roger Kenyon said. "But actually detecting a source is hard. But if we are to make a difference and prevent further damage to Lake Erie, we need to demonstrate that phosphorus is a problem and set goals. "First we have to get serious about how phosphorus is getting into lake Erie, then do whatever it takes to limit it," Marsh said. Culver added: "There is no way to remove zebra or quagga mussels from the lake. I recommend increased efforts in monitoring inputs of nutrients, especially phosphorus and nitrogen into the lake." Because, just as DDT proved to be beneficial to our way of life, it resulted in devastating damage to our natural resources. Phosphorus might do the same.
|
